Marcelle Malan looks at intensity as a key variable in exercise for mental health, asking does low intensity or higher intensity exercise lead to improved outcomes?
Despite extensive evidence for the importance of exercise for mental health and its key role in the prevention and management of psychiatric disorders, exercise is often brushed over in mental health care. Development of exercise prescription guidelines is also complicated by the considerable variation in the presentation of psychiatric disorders and significant gaps in the knowledge base.4
There has been a general trend in both psychiatry and psychology towards integration of neuroscience and neurophysiology into mental health practice frameworks. This integration of fields of study has broadened understanding of the interconnected nature of psychological and physiological factors in neuropsychiatric disorders.1
At the same time, advances in technology have allowed nosey parkers in lab coats to have a deeper look into cellular energetics, particularly the mechanisms underlying metabolic dysfunction.2,3 It has been known for some time now that metabolic dysfunction is a key driver for cognitive decline and neurodegenerative conditions such as Alzheimer’s disease. It is becoming clear that this cascade of mechanisms also mediates the relationship between metabolic health and neuropsychiatric disorders such as major depressive disorder, bipolar disorder, anxiety disorder and schizophrenia. 6,8
Linking metabolic and neuropsychiatric health
Possibly the first evidence to link metabolic health and mental health was research showing a correlation between depressive symptoms and chronic metabolic inflammation. 6 It is known that people with neuropsychiatric disorders tend to have poorer cardiometabolic health. The logical assumption is that psychiatric ill health drives adverse lifestyle factors such as inactivity, poor diet, smoking, etc – not that poor metabolic health is itself a driver for the development of psychiatric disorders. But, as it turns out, the relationship is bidirectional. 6,8,9
There is an extensive literature implicating chronic metabolic inflammation as a direct risk factor for the development of depression and bipolar disorder. Dysregulation of cardiometabolic function is part of the pathophysiology of schizophrenia, and anxiety disorders are associated with dysregulated glucose metabolism. 6,8,9,11,14
In addition, both major depressive disorder and bipolar disorder are associated with higher levels of circulating pro-inflammatory markers and lower levels of circulating anti-inflammatory markers. Neuro-inflammation-associated brain alterations have also been reported in anxiety disorders, likely mediated by the stress response. Altered immune function is also a hallmark of schizophrenia, with implications for exercise prescription. 14
“Exercise is the only known intervention for improving mitochondrial health, driving adaptation and resilience”
Finally, neuropsychiatric conditions are associated with dysregulation of several neurotransmitter pathways, including the noradrenergic system, the serotonergic system and the dopaminergic system. There are many mechanisms which link cellular signalling (neurotransmitter pathways) and neuronal metabolism – metabolic stress upregulates levels of neurological inflammation, in turn affecting neuronal signalling. 8,10
The classification of psychiatric disorders into distinct, if somewhat overlapping, diagnoses is necessary for appropriate treatment. However, the sole focus on diagnostic distinctions obscures evidence that many neuropsychiatric disorders have common: underlying biological drivers which connect cellular energetics (metabolic function), cellular signalling (neurotransmitter pathways) and immune function.
Figure 1: A complex interplay of cellular energetics, cellular signalling and immune function – mediated by the stress response – drives the pathophysiology of both neuropsychiatric disorders and metabolic disease
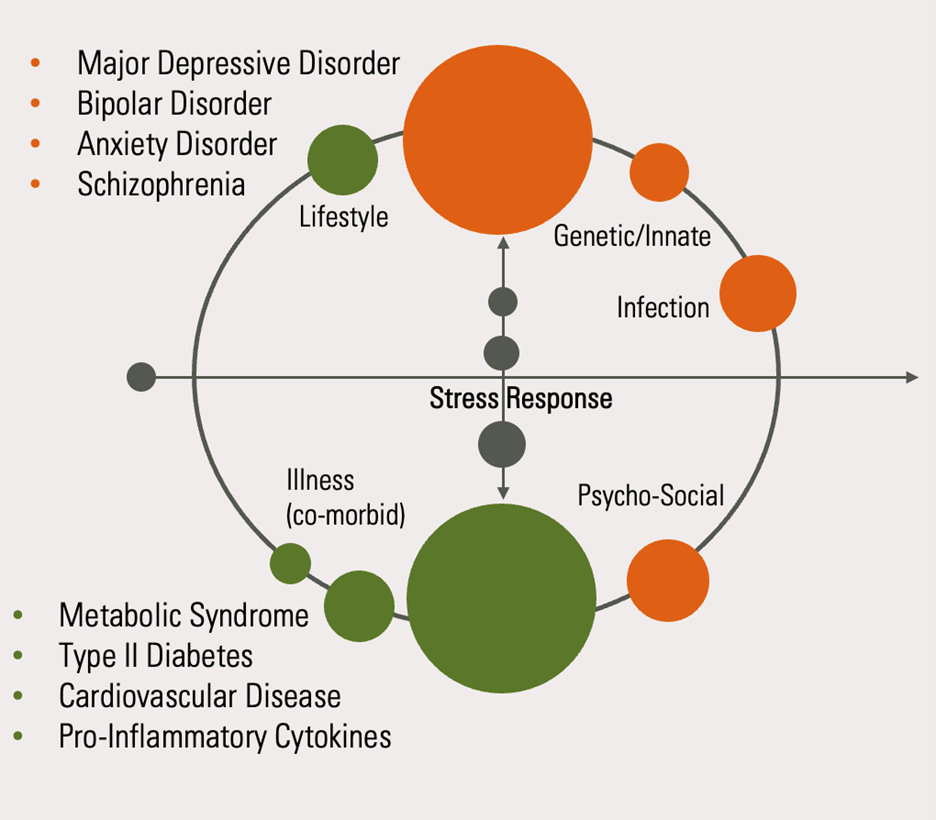
Chronic dysregulation of these functions upregulates metabolic inflammation which, when combined with a genetic predisposition towards psychiatric illness and environmental triggers, drives the pathophysiology which underpins the development of neuropsychiatric disorders. These physiological drivers are highly modulated by chronic psycho-emotional stress, particularly in early life.
Chronic stress is highly implicated in the dysregulation of metabolic function. The purpose of the acute stress response is to facilitate the bioenergetic mechanisms that enable mobilisation of the body. If a person with a knife is running towards you, you want a bunch of glucose dumped in your bloodstream, so you have the energy to trip up that so-and-so. Or run away. You do you. This acute response is adaptive – it gets you moving. When stressors become chronic or very severe, it leads to maladaptive responses in both autonomic regulation and metabolic regulation.
A complete lack of any stressors will also lead to maladaptation – sedentary behaviour is a principal driver of metabolic disease. Intermittent and appropriately dosed stressors – such as exercise – are vital for metabolic health.
Mitochondria – The linchpin of metabolic health
The linchpin of cellular energy metabolism is the mitochondrion. Mitochondria are tiny structures found inside all cells (except red blood cells). Colloquially referred to as the “powerhouses of the cell”, they produce the molecule adenosine triphosphate (ATP) through the process of oxidative phosphorylation. ATP is required for bioenergetics and, by extension, for life itself. The ability of the mitochondria to provide aerobic energy to otherwise anaerobic cells have allowed those cells to generate new genes and proteins, driving the evolution of mammals. 11
The highest density of mitochondria in the body are found in skeletal muscle and in the brain, due to the high energy demands of these tissues. Skeletal muscle is the principal driver of the body’s aerobic capacity. In addition to producing energy, we know that skeletal muscle also acts as an endocrine organ, and that there is active chemical crosstalk between muscle and brain.
Mitochondria in neurons and glial cells in the brain are critical to the function of these cells, impacting energy availability, cellular signalling and neuro-immune responses. Dysregulated mitochondrial function impacts neuroplasticity and neurological inflammation.
There are myriad factors which influence mitochondrial function, including genetic variation, lifestyle factors, stress, etc. However, exercise is the only known intervention for improving mitochondrial health, driving adaptation and resilience. This relationship is sharply mediated by nutrition, sleep and stress management. 10,11
Mitochondrial dysfunction results in a reduced capacity for fatty acid oxidation (FatOx). This leads to lower overall functional capacity and necessitates a greater reliance on glycolysis/anaerobic respiration – even at low exercise intensities. This compromise in bioenergetics leads to dyslipidaemia, insulin resistance, higher levels of oxidative stress, elevated immune markers and chronically elevated blood lactate (given that lactate is a by-product of glycolysis). 3,11
Figure 2: The mitochondrial dysfunction cascade
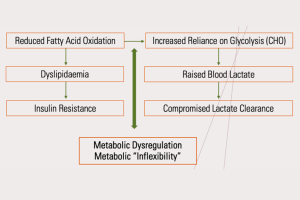
The brain is particularly vulnerable to mitochondrial dysfunction. Increased oxidative stress drives chronic neuro-inflammation. Excess accumulation of lactate, a hallmark of mitochondrial dysfunction, is often seen in both in the cerebrospinal fluid and in the blood of those with neuropsychiatric illness.
Exercise to promote metabolic rehabilitation should aim to:
- optimise oxidative phosphorylation and oxidation of fatty acids, thereby reducing oxidative stress and metabolic inflammation
- promote structural repair of existing mitochondria and the generation of new mitochondria (mitochondrial biogenesis)
- facilitate the ability of cells to metabolise lactate as a fuel source and harness the strength of lactate as a signalling molecule which mediates the intensity-dependent adaptations to exercise.

Exercise intensity for metabolic rehabilitation
The popular concept that the ‘best’ exercise intensity for mitochondrial health is Zone 2 (using the common 6 Zone Model) is based on the observation that this intensity seems to maximise fat oxidation.
While there is good evidence for this, that evidence comes primarily from research done on elite and sub-elite endurance athletes. Athletes typically do a very high volume of training and, in research on Zone 2 training, the required minimum amount of aerobic exercise suggested is around four hours per week. The general population, and certainly clinical populations, does not do anywhere near this volume of aerobic conditioning. 11,12
There is clear evidence for a dose response in applied research on exercise for neuropsychiatric conditions, with exercise of at least moderate intensity yielding better outcomes than low-intensity exercise. 1,4 It is quite clear that a low volume of exercise at a relatively low intensity such as Zone 2 is unlikely to drive significant adaptations on its own (with the caveat that if someone is completely sedentary, ANY volume of exercise at any intensity is going to be beneficial).
“Increased oxidative stress drives chronic neuro-inflammation.”
Mitochondrial dysfunction is characterised both by a compromise in oxidative capacity and a compromise in the capacity to metabolise lactate. As such, it is reasonable to argue that exercise intensity targeting metabolic rehabilitation (in the 6 Zone model), should span the whole of the area which lies between Zone 2 and Zone 4 – the entire ‘Lactate Threshold’. This means that at least some of our exercise needs to be at least at a moderate level of intensity (Zone 4). This is consistent with the dose-response we tend to see in the applied research.
In the 6 Zone Model, Zone 4 corresponds to the anaerobic threshold or the Maximal Lactate Steady State (MLSS). This is the intensity at which an individual can sustain exercise without an exponential increase in blood lactate. Typically, the blood lactate concentration is around 2mmMol/L at Zone 2 and 4mmMol/L at Zone 4. For someone who is metabolically compromised, blood lactate can already reach 2mmMol/L at rest.
As such, it is highly desirable to personalise exercise intensity by directly measuring blood lactate during a graded exercise assessment. If this is not possible, intensity can be approximated by using heart rate and rate of perceived exertion, where Zone 2 would be around 65% of HRmax and Zone 4 at around 85% of HRmax – although this intensity may be too much for some people to begin with.
Figure 3: Typical FaxOX and lactate responses at different exercise intensities
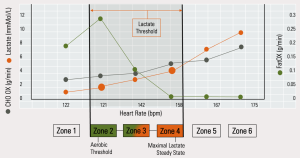
In its capacity as an exerkine, lactate mediates the intensity-dependent response to exercise, being highly correlated to the release of the signalling molecule brain-derived neurotrophic factor (BDNF). Lactate is also a key fuel source for the brain and drives a multitude of adaptive responses throughout the entire body. 12, 7
In research on exercise and mental health, moderate to higher intensity exercise generally leads to improved outcomes over low-intensity exercise, but high-intensity interval training does not outperform moderate-intensity exercise. So, the goldilocks principle very much applies.
Schizophrenia tends to be an exception to this general rule on dose dependence. As mentioned previously, schizophrenia is associated with cardiometabolic and immune dysfunctions which can lead to significant exercise intolerance. As a rule, higher intensity exercise will tend to lower immune markers over time. This sometimes seems to be the opposite in schizophrenia.
Some people with schizophrenia also exhibit autonomic dysregulation, which results in chronotropic insufficiency – an inability of the heart rate to increase effectively in response to exercise. This does not change with exercise. This leads to significant exercise intolerance. These individuals must be assessed where possible to personalise intensity. In general, Zone 2 exercise will be the best option, along with some strength training if possible. 5,9,13
For those with anxiety disorders, modulation of the stress response is likely to be a major consideration. There is some relationship between blood lactate concentration and state anxiety and, for some individuals, exercise at or near the MLSS may provoke symptoms. Exercise-induced anxiety can be very frustrating and will most likely require a graduated approach with coaching on autonomic regulation techniques.
Personalisation based on physiological measures, subjective perceived exertion and personal enjoyment is optimal.
As a final note, there is very little consensus with regards to resistance training intensity/load in psychiatric populations; however, from research on brain health and cognitive function, it seems that an intensity corresponding to 10-12 repetitions (60-80% 1RM) optimises both myokine release and functional capacity for daily living.
Check out Marcelle Malan’s new online CPD course Exercise and Neuropsychiatric Health to learn more
References and Further Reading:
- Alsuwaidan MT, Kucyi A & Law CWY (2009), Exercise and bipolar disorder: A review of neurobiological mediators, Neuromolecular. https://pubmed.ncbi.nlm.nih.gov/19649751/
- Sethi S & Brietzke E (2016), Omics-based biomarkers: Application of metabolomics in neuropsychiatric disorders, Int J Neuropsychopharmacol. https://pmc.ncbi.nlm.nih.gov/articles/PMC4815467/
- Erjavec GN et al (2018), Short overview on metabolomic approach and redox changes in psychiatric disorders, Redox Biology.
- Heissel A et al (2023), Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression, Br J Sports Med, 57: 1,049-57.
- Herbsleb M et al (2019), The influence of continuous exercising on chronotropic incompetence in multi-episode schizophrenia, Front Psychiatry, 10: 90.
- Pan A et al (2012), Bidirectional association between depression and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies, Diabetes.
- Park HJ, Rhie SJ & Shim I (2022), Regulatory role of cytokines on etiology of depression in animal models: Their biological mechanisms and clinical implication with physical exercise, J
Exerc Rehabil., 18: 344-49. - Sun L et al (2022), Physical exercise and mitochondrial function: New therapeutic interventions for psychiatric and neurodegenerative disorders, Frontiers in Neurology.
- Anglin RE & Tarnopolsky MA (2012), The psychiatric presentation of mitochondrial disorders in adults, J Neuropsychiatry Clin Neurosci.
- Sorriento D, Vaia ED & Iaccarino G (2021), Physical exercise: A novel tool to protect mitochondrial health, Frontiers in Physiology.
- San-Millán I (2023), The key role of mitochondrial function in health and disease, Antioxidants.
- San-Millán I et al (2018), [C] Relationships Between Vo 2 max and Blood Lactate During Exercise Across Different Populations: 1128 May 31 9: 15 AM-9: 30 AM. Medicine & Science.
- Pietrangelo T, Filippo ESD & Mancinelli R (2015), Low-intensity exercise training improves skeletal muscle regeneration potential. Frontiers in …, Front Physiol.
- Won E & Kim YK (2020), Neuroinflammation-associated Alterations of the Brain as Potential.









